2021 Immunization Updates: COVID-19, Influenza, and Meningococcal Disease
 Each year, the California Medi-Cal Drug Use Review (DUR) program issues an annual summary of updates on immunization guidelines, products, and/or research in collaboration with the California Department of Public Health (CDPH) Immunization Branch. For reference, the recommended immunization schedules for 2021 in the United States can be accessed on the Centers for Disease Control and Prevention (CDC) website:
Each year, the California Medi-Cal Drug Use Review (DUR) program issues an annual summary of updates on immunization guidelines, products, and/or research in collaboration with the California Department of Public Health (CDPH) Immunization Branch. For reference, the recommended immunization schedules for 2021 in the United States can be accessed on the Centers for Disease Control and Prevention (CDC) website:
Learning Objectives:
- Describe the vaccines currently available to prevent infection with SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19)
- Discuss strategies for improving (COVID-19) vaccination rates and vaccine confidence
- Review updated Advisory Committee on Immunization Practices (ACIP) recommendations for COVID-19, influenza, and meningococcal disease vaccines
COVID-19 Vaccine General Recommendations
COVID-19 vaccination is currently recommended for all people 12 years of age or older to prevent infection with SARS-CoV-2, the virus that causes COVID-19. During 2020, the following three COVID-19 vaccine products were US Food and Drug Administration (FDA) authorized and are recommended for use in the U.S:
- A messenger RNA (mRNA) vaccine, BNT162b2 by Pfizer-BioNTech (for people 12 years of age or older) – a two-dose primary series, separated by 21 days
- An mRNA vaccine, mRNA-1273 by Moderna (for people 18 years of age or older) – a two-dose primary series, separated by 28 days
- A recombinant vector vaccine, Ad26.CoV2.S by Johnson & Johnson/Janssen (for people 18 years of age or older) – single dose
On December 11, 2020, the first mRNA COVID-19 vaccine became available under emergency use authorization (EUA) for individuals 16 years of age and older, which was later expanded to include ages 12 through 15 years. As defined by the FDA, EUAs can be used during public health emergencies to provide access to medical products that may be effective in preventing, diagnosing, or treating a disease, provided that the FDA determines that the known and potential benefits of a product, when used to prevent, diagnose, or treat the disease, outweigh the known and potential risks of the product. On August 23, 2021, the FDA issued full approval of the PfizerBioNTech mRNA COVID-19 vaccine for use in people aged 16 years and older.
The CDC does not recommend one vaccine over another. All recommended COVID-19 vaccines lead to expression of the SARS-CoV-2 spike glycoprotein in cells, but there are important differences in their mechanism and composition. The mRNA vaccines are lipid-nanoparticleformulated, nucleoside modified vaccines that encode for the spike glycoprotein. The recombinant vector vaccine uses a replication incompetent adenovirus to express the spike protein in cells. A person is considered fully vaccinated two weeks after completion of the primary vaccine series. In California, COVID-19 vaccine appointments can be made online at https://myturn.ca.gov/.
Viral mutations in the amino acid sequences of the SARS-CoV-2 spike protein can introduce new variants with evolutionary advantages. Currently, it is estimated that over 99% of U.S. COVID-19 cases are due to a single, highly transmissible variant of the SAR-CoV-2 virus, known as
B.1.617.2 (Delta variant). The enhanced transmission of the Delta variant is associated with spike protein mutations at the receptor binding domain, the furin cleavage site, and the antigen supersite. COVID-19 vaccines are effective at protecting against severe disease and death from currently circulating SARS-CoV-2 variants, including the Delta variant.
COVID-19 Vaccination in the Medi-Cal Population
The Medi-Cal program serves a diverse population of approximately 14 million people, including individuals that face health disparities. As of August 22, 2021, only 50.7% of eligible Medi-Cal enrollees have received at least one dose of a COVID-19 vaccine compared with 75.8% of Californians as a whole. Improving immunization rates among eligible Medi-Cal enrollees is essential to prevent further health inequities in California’s most vulnerable communities. Several large groups of Medi-Cal beneficiaries have been identified as being disproportionately challenged during the COVID-19 vaccination distribution, including the homebound, people 50 – 64 years of age with multiple chronic diseases, communities of color, and youth 12 – 25 years of age.
To help remedy this situation, DHCS announced a vaccination incentive program to improve vaccination rates and expand access for disadvantaged groups in the Medi-Cal population. Starting September 1, 2021, $350 million has been allocated for managed care plans (MCPs) to earn incentive payments for COVID-19 vaccination outreach activities. For more information, providers and plans can review the Medi-Cal COVID-19 Vaccination Incentive Program (All Plan Letter 21010 (Revised), which is available on the DHCS website.
COVID-19 Vaccination Considerations Involving Pregnancy, Lactation, and Fertility
COVID-19 vaccination is recommended for people who are pregnant, breastfeeding, trying to become pregnant, or have plans for pregnancy in the future. Pregnant people are at an increased risk for hospitalization and preterm birth after contracting COVID-19, underscoring the importance of vaccination. Notably, a recent study found COVID-19 vaccination during pregnancy may provide protection from SARS-CoV-2 to neonates. ACIP also emphasized in their recommendations that there is no data showing COVID-19 vaccines cause infertility.
Any FDA-authorized vaccine can be given during pregnancy, but people who are pregnant should be informed of the risk of thrombosis with thrombocytopenia syndrome (TTS) after receipt of the Johnson & Johnson/Janssen COVID-19 vaccine and the availability of other COVID-19 vaccine options for which this risk has not been observed.
The CDC’s Toolkit for Pregnant People and New Parents provides a comprehensive collection of educational resources regarding COVID-19 vaccine considerations related to pregnancy, fertility, and lactation. In addition, CDPH has published Guidance for Vaccination during Pregnancy, which is available on the CDPH website.
COVID-19 Vaccination Considerations for Immunocompromised People
ACIP also provides guidance for use of COVID-19 vaccines in immunocompromised people, including assessment of immune competency and dosing recommendations. Any person with immunocompromised status is at an increased risk of serious, prolonged illness due to COVID-19 and is recommended to be vaccinated for COVID-19.
Recent evidence has shown a reduced immune response in this population following the primary mRNA COVID-19 vaccine series, resulting in the ACIP recommendation on August 13, 2021, supporting the use of a third dose of mRNA COVID-19 vaccine in patients with moderate to severe immunocompromised status. As defined by ACIP, individuals can be moderately to severely immunocompromised due to a medical condition or immunosuppressive medication regimen, including – but not limited to – primary immunodeficiency, advanced HIV infection, active treatment for malignancy, or history of solid-organ transplant with current immunosuppressive therapy. If a third dose is determined to be appropriate, ACIP recommends it should be given at least 28 days after completion of the COVID-19 mRNA vaccine series, and an attempt should be made to administer the same product as the vaccine given for the first two doses. For more information, providers can refer to COVID-19 Vaccine Indications for Patients Who Are Immunocompromised, which is available on the CDC website.
Due to limited data, individuals who received the Johnson & Johnson/Janssen COVID-19 vaccination are not recommended to receive an additional dose at this time. Again, providers are encouraged to refer to the Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States for the most current information on recommendations for immunocompromised people.
For help discussing the benefits and risks of receiving a third dose of COVID-19 vaccination, providers can visit How to Talk with Patients Who Are Immunocompromised: Discussing an Additional Dose of an mRNA COVID-19 Vaccine, which is available on the CDC website. Clinicians should discuss the potential for reduced immune response following COVID-19 vaccines with immunocompromised patients and reinforce prevention measures, even after receiving an additional dose of vaccine.
COVID-19 Vaccination Considerations for Children and Adolescents
Currently, the mRNA vaccine by Pfizer-BioNTech is the only vaccine product approved for individuals 16 years of age and older. The vaccine also continues to be available under EUA for individuals 12 through 15 years of age. As of July 31, 2021, a recent CDC assessment found that only 41.2% of eligible adolescents in California have completed their COVID-19 vaccination series. With surging COVID-19 cases from the Delta variant and the return to in-person learning, improving vaccination rates among all eligible students in this group is especially important.
The American Academy of Pediatrics (AAP) in their policy statement COVID-19 Vaccines in Children and Adolescents recommends the following:
- COVID-19 vaccination for all children and adolescents 12 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age.
- Any COVID-19 vaccine authorized through Emergency Use Authorization by the US Food and Drug Administration, recommended by the CDC, and appropriate by age and health status can be used for COVID-19 vaccination in children and adolescents.
- Given the importance of routine vaccination and the need for rapid uptake of COVID-19 vaccines, the AAP supports coadministration of routine childhood and adolescent immunizations with COVID-19 vaccines (or vaccination in the days before or after) for children and adolescents who are behind on or due for immunizations (based on the CDC and AAP Recommended Child and Adolescent Immunization Schedule) and/or at increased risk for vaccine-preventable diseases.
In April 2021, cases of myocarditis and pericarditis after mRNA COVID-19 vaccination were reported. The occurrence of myocarditis and pericarditis is rare, with 39-47 expected cases per million second doses of mRNA COVID-19 vaccine. Reassuringly, patients who presented with myocarditis and pericarditis responded promptly to medications and rest. Additionally, recent data has identified COVID-19 infection as a significant risk factor for development of myocarditis, as described in the Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data — United States, March 2020–January 2021, published in the Morbidity and Mortality Weekly Report (MMWR), which is available on the CDC website. After adjusting for patient and hospital characteristics, this report found that patients with COVID-19 during March 2020 – January 2021 had, on average, 15.7 times the risk for myocarditis compared with those without COVID-19, with the risk increasing to greater than 30.0 times the risk among patients younger than 16 years of age.
After a benefit-and-risk assessment, ACIP determined that the potential benefit of vaccination greatly outweighs the potential risk of myocarditis and pericarditis. However, providers should counsel patients receiving mRNA COVID-19 vaccines, particularly young adults, on the signs and symptoms of myocarditis and pericarditis after vaccination (chest pain, shortness of breath, or palpitations).
Strategies to Improve COVID-19 Vaccination
Among those who have not been vaccinated against COVID-19, eight in ten say they plan to turn to doctors, nurses, and other health providers before deciding whether to get COVID-19 vaccination. To support all California health care providers in motivating patients to receive the COVID-19 vaccine, CDPH launched the 30 Conversations in 30 Days campaign. The goal of the campaign is to help equip all health care providers with tools and techniques to proactively talk with their patients about the merits of the COVID-19 vaccine and help them make a vaccine appointment. A comprehensive slide deck entitled, “How to Talk to Your Patients about COVID-19 Vaccine” is available from CDPH that provides guidance on how to initiate conversations and communicate key messages about COVID-19 vaccination to patients. The CDC also offers suggestions on motivational interviewing and other effective tools on their Talking with Patients about COVID-19 Vaccine webpage.
Influenza Vaccine
As in prior years, routine annual influenza vaccination is recommended for everyone 6 months of age or older without contraindications. For the upcoming influenza season, widespread influenza vaccination will be critical to reduce the impact of respiratory illnesses in the population and the resulting burdens on the healthcare system during the COVID-19 pandemic. Any reduction in flu cases and their severity could make a difference for hospital capacity.
For the 2021 – 2022 season, inactivated influenza vaccines (IIVs), recombinant influenza vaccine (RIV4), and live attenuated influenza vaccine (LAIV4) are expected to be available. For the first time, all available influenza vaccines for the 2021 – 2022 season are quadrivalent.
U.S. IIV4s and LAIV4 (egg-based) influenza vaccines contain hemagglutinin (HA) derived from the following influenza viruses:
- A/Victoria/2570/2019 (H1N1) pdm09-like virus (different strain from last season)
- A/Cambodia/e0826360/2020 (H3N2)-like virus (different strain from last season)
- B/Washington/02/2019-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage)
U.S. cell culture–based inactivated (ccIIV4) and RIV4 influenza vaccines contain HA derived from the following influenza viruses:
- A/Wisconsin/588/2019 (H1N1) pdm09-like virus (different strain from last season)
- A/Cambodia/e0826360/2020 (H3N2)-like virus (different strain from last season)
- B/Washington/02/2019-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage)
ACIP has published updated guidance for use of COVID-19 vaccines during the 2021 – 2022 influenza season. Current recommendations permit the coadministration of COVID-19 and influenza vaccines. Providers are encouraged to check the Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States for updates throughout the influenza season.
Early vaccination (as soon as there is vaccine available) should now be considered for women in the third trimester of pregnancy and in children 6 months through 8 years of age who need two doses of influenza vaccine. The American College of Obstetricians and Gynecologists (ACOG) and ACIP recommend women who are pregnant or plan to become pregnant during flu season receive any of the recommended inactivated influenza vaccines. Of note, LAIV administration during pregnancy is contraindicated.
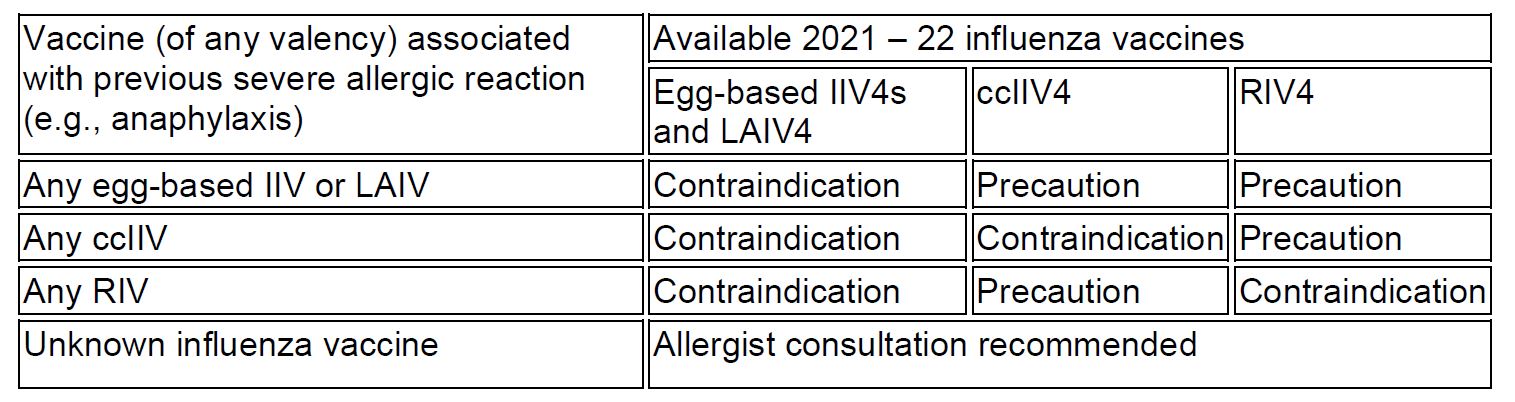
Egg allergy is now considered a precaution, not a contraindication, to IIV administration. As shown in Table 1, severe allergic reaction (e.g., anaphylaxis) after a previous dose of any eggbased IIV or LAIV, any ccIIV or any RIV is a contraindication to egg-based IIV4s and LAIV4. Severe allergic reaction to any ccIIV is also considered a contraindication to ccIIV4 and a precaution to RIV4. Severe allergic reaction to any RIV is a contraindication to any egg-based IIV4s and LAIV4 and RIV4, and a precaution to ccIIV4. For severe allergic reaction occurring with an unknown influenza vaccine, consultation with an allergist is recommended.
Table 1: Contraindications and Precautions for Persons with a History of Severe Allergic Reaction to an Influenza Vaccine*
∗ Table was taken from the Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2021-2022 Summary of Recommendations, published in the MMWR, which is available on the CDC website.
In March 2021, the FDA also expanded the age indication for the use of ccIIV4 to include children aged 2 years and older. For additional information about available formulations, dosing of influenza vaccine, recommendations for the timing of vaccination, and additional contraindications for the use of LAIV4, providers may refer to the Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2021 – 2022 Influenza Season, published in the MMWR, which is available on the CDC website.
For patient education materials on influenza vaccines, CDPH offers patient educational materials at the Fight Flu Together available from the CDPH website. The Communication Resource Center and 2021 – 2022 Influenza Vaccination Talking Points created by CDPH and the California Immunization Coalition (CIC) provide communication toolkits for healthcare professionals to use during the 2021-2022 season.
Meningococcal Vaccine
A September 2020 report summarized previously published recommendations from ACIP regarding the prevention of meningococcal disease in the United States and included new recommendations for administration of booster doses of serogroup B meningococcal (MenB) vaccine for persons at increased risk.
ACIP now recommends a MenB booster dose for individuals 10 years of age or older who remain at increased risk of serogroup B disease, including people with complement deficiency, complement inhibitor use, asplenia, or microbiologists routinely exposed to N. meningitidis isolates. A booster dose should be administered one year after completion of the primary series, with an additional booster dose every two to three years thereafter. A single MenB booster dose is also recommended for patients at increased risk during an outbreak if it has been over one year since primary series completion. Of note, while either MenB vaccine can be given, the same product must be used for all doses in a series, including booster doses. MenB vaccination is also recommended for persons aged 16 through 23 years (preferred age 16 through 18 years) on the basis of shared clinical decision-making.
In addition, a new tetanus toxoid conjugate vaccine for meningococcal groups A, C, W, and Y (MenACWY-TT) for the prevention of meningococcal disease caused by serogroups A, C, W, and Y in persons two years of age or older was licensed in 2020. For additional information about all U.S.-licensed MenACWY and MenB vaccines, providers may refer to the Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020, published in the MMWR, which is available on the CDC website.