Effective 2/21/2017, the Pharmacy and Therapeutics Committee has approved the following changes:
| Date: | December 21, 2016 |
| To: | Health Plan of San Joaquin (HPSJ) Providers and Practitioners |
| From: | HPSJ Medical Management Department |
| Subject: | Comprehensive Tobacco Cessation Services |
| Business: | Medi-Cal, MCAP (Medi-Cal Access Program, previously known as AIM) |
Additions to the Formulary:
- Potassium Citrate ER 15 mEq (Urocit-K): No PA required.
- Alemtuzumab (Lemtrada): PA required. Restricted to neurologists.
- Documented diagnosis of relapsing remitting multiple sclerosis.
- History of inadequate treatment response to 1 drug in the following 2 categories:
- Betaseron / Avonex / Rebif / Copaxone
- Gilenya / Aubagio / Tecfidera
- No previous history of malignancy
- Negative test results for Active/Latent Infection (HIV, HBV, HCV, or TB)
- Daclizumab (Zinbryta): PA required. Restricted to neurologists. Zinbryta is reserved for
members with:- Documented diagnosis of relapsing remitting multiple sclerosis.
- History of inadequate treatment response to 1 drug in the following 2 categories:
- Betaseron / Avonex / Rebif / Copaxone
- Gilenya / Aubagio / Tecfidera
- No previous history of malignan
- Negative test results for Active Infection (HIV, HBV, HCV, or TB)
- Glatiramer Acetate (Glatopa): PA required. First line therapy for MS. Must be prescribed by a neurologist, for verified diagnosis of multiple sclerosis. Restricted to Specialty Pharmacy.
- Melatonin: No PA required.
- Fleet Enema: No PA required.
- Naloxegol (Movantik): PA required. Movantik is reserved for members with:
a. Documented diagnosis of opioid-induced constipation with chronic non-cancer pain
b. Treatment failure of dose-optimized, regularly scheduled polyethylene glycol for 2 months (as evidenced by prescription history fills) AND two of the following: bisacodyl, Senna, lactulose, magnesium citrate or hydroxide. - Methylnaltrexone (Relistor): PA required. Relistor is reserved for members with:
a. Documented diagnosis of opioid-induced constipation with chronic non-cancer pain
b. Treatment failure of naloxegol (Movantik) and lubiprostone (Amitiza).
c. Patient must have also failed regularly scheduled, dose optimized polyethylene glycol (Miralax), AND two of the following: bisacodyl, Senna, lactulose, psyllium, magnesium citrate or hydroxide.
d. For subcutaneous Relistor injection, patient must also have documented inability to swallow tablets/capsules. - Granisetron Tablet (Kytril): PA required. Reserved for patients with documented treatment
failure of dose optimized Ondansetron therapy. - Rizatriptan 10 mg ODT (Maxalt): Limited to 9 tablets per month.
- Quillivant ER 20 mg Chewables: No PA required.
- Quillivant XR 25mg/5mL suspension: No PA required.
- Lanthanum Carbonate 750mg Tablets (Fosrenol): PA required. Third line therapy for patients with treatment failure of calcium acetate and sevelamer. For Fosrenol 500mg, 750mg tablets, limited to 3 tablets per day.
- Patiromer (Veltassa): PA required. Veltassa is reserved for members with clinical notes documenting both of the following:
a. Diagnosis of heart failure, OR hypernatremia, OR risk for colonic necrosis (impaction, chronic constipation, inflammatory bowel disease, ischemic colitis, vascular intestinal atherosclerosis, bowel obstruction)
b. Potassium (K+) > 5.5 mEq/L - Olodaterol hydrochloride (Striverdi Respimat): Step therapy to concurrent use of ICS is required. Limit 1 package per month.
- Invokamet XR (Canagliflozin/Metformin) 50mg/500mg, 150mg/500mg, 50mg/1000mg,
150mg/1000mg tablets: Limited to two tablets per day. - Selexipag (Uptravi): PA Required. Uptravi is reserved for patient’s who meet all of the following
criteria:- WHO Group I and PAH Functional Class III
- (-) vasoreactivity test OR (+) vasoreactivity test and use of CCB (if appropriate)
- Inadequate response to dose optimized use of PDE-5 inhibitors and endothelin receptor
antagonists.
Formulary Status Changes:
- Lubiprostone (Amitiza): PA required. Amitiza is reserved for members who have failed treatment with linaclotide (Linzess) or naloxegol (Movantik). Patient must have also failed regularly scheduled, dose optimized polyethylene glycol (Miralax), AND two of the following: bisacodyl, Senna, lactulose, psyllium, magnesium citrate or hydroxide.
- Testosterone: Testosterone therapy is reserved for patients undergoing Gender Transition, or with documentation of hypogonadism as evidenced by testosterone levels below 300ng/dL confirmed on two separate occasions with levels drawn before 10:00am.
- Granisetron Patch (Sancuso): PA required. Reserved for members with documented inability to take medications by mouth, including ODT formulations.
- Dolasetron (Anzemet): PA required. Reserved for treatment failure of Ondansetron and Granisetron Tablets
- Palonosetron (Aloxi): PA required. Reserved for use in patients receiving highly-emetogenic chemotherapy, with treatment failure of ondansetron, or documented inability to swallow.
- Daytrana patches: PA required. Restricted to patients with documented inability to swallow tablets, capsules, chewables, solutions, or suspensions.
- Iron Sucrose (Venofer): PA required. Documentation of the following is required:
- Currently on dialysis
- Recent labs showing TSAT ≤30%
- AND ferritin ≤500 ng/mL
- OR currently not on dialysis
- Documentation that use of less invasive iron supplementation is not sufficient for treatment of the patient’s iron deficiency
- Current labs showing TSAT ≤30% and ferritin ≤500 ng/mL
- Note: Iron sucrose (Venofer) is approved 3 months at a time. Submission of TSAT and
Ferritin levels with the renewal request is required showing TSAT ≤30% and ferritin ≤500
ng/mL.
- Currently on dialysis
- Lanthanum Carbonate 500 mg tablets (Fosrenol): PA required. Reserved for treatment failure of calcium acetate and sevelamer. For Fosrenol 500mg, 750mg tablets, limited to 3 tablets per day.
- Cinacalcet (Sensipar): PA Required. Indicated for patients with secondary hyperparathyroidism with BiPTH > 200 pg/ml despite compliant use of phosphate binders OR patients with CKD-5D. Limited to 2 tablets per day for Sensipar 30mg, 60mg tablets.
- Mepolizumab (Nucala): PA required. Reserved for patients with:
- Poorly controlled, severe eosinophilic asthma with baseline serum eosinophil counts of either ≥ 150 cells/µL at initiation of treatment or ≥ 300 cells/µL in the past 12 months
- AND 2 or more exacerbations in the past 12 months, despite being compliant with doseoptimized
- i. [1] Inhaled Corticosteroids (ICS) + Long-Acting Beta-2 Agonist (LABA)
- ii. [2] Spiriva Respimat, and
- iii. [3] leukotriene modifier or theophylline.
- Must be prescribed by an allergist.
- Note: Initial approval is 6 months. Continuing approval will require updated clinic notes with documented therapeutic response in the form of improved symptomology.
- Zolmitriptan (Zomig): Zolmitriptan is reserved for treatment failure to either Sumatriptan or Rizatriptan in the last 365 days.
- Eletriptan (Relpax): Eletriptan is reserved for treatment failure to [1] Sumatriptan or Rizatriptan AND [2] Naratriptan or Zolmitriptan in the last 365 days.
- Almotriptan (Axert): Almotriptan is reserved for treatment failure to [1] Sumatriptan or Rizatriptan AND [2] Naratriptan or Zolmitriptan in the last 365 days.
- Calcium Acetate Capsules, Gelcaps: PA Required. Reserved for treatment failure of Calcium Acetate Tablets.
- Crotamition (Eurax) 10 % Cream/Lotion: Reserved for patients with treatment failure of 2 treatments of Permethrin 5% cream within the past 30 days as evidenced by prescription fill history.
Deletions from the Formulary:
The following products will be removed from the formulary as of February 27, 2017:
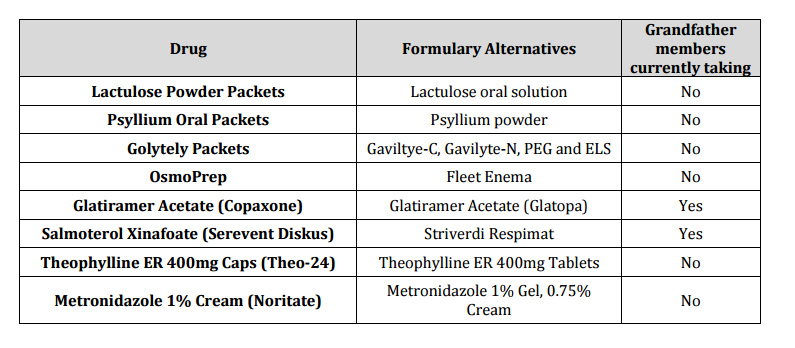
Health Plan of San Joaquin is dedicated to providing all members the best health care available in the most effective and efficient manner. We believe that this change in our Pharmacy Drug Benefit will not affect the quality of the care you provide.
You may contact our Provider Services Department with any questions or concerns Monday through Friday 8:00 am to 5:00 pm at (209) 942-6340 or 1-888-936-PLAN (7526).
Thank you for your continued support of Health Plan of San Joaquin

